Catégories
- De nouvelles Haomei (311)
le pharmaceutical tablets packaging design must balance drug protection (éprouvant de l'humidité, résistant à l'oxydation, résistant à la lumière, etc.), facilité d'utilisation et conformité réglementaire. Différentes structures nécessitent différentes combinaisons de matériaux.
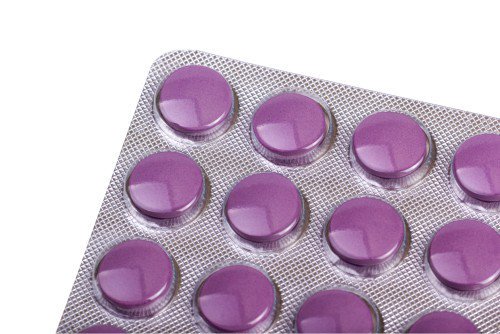
Les comprimés pharmaceutiques sont généralement emballés dans des packs blister ou des packs à bande.
1. Blister Pack
This is one of the most commonly used tablet packaging formats. Il se compose d'une coquille de cloques et d'un couvercle. Its characteristic is that each tablet is individually sealed within a “blister.”
Structural Components and Materials:
– Blister Shell (Cavity)
Fonction: Contains the tablets, creating an independent, sealed space.
Common Materials: Chlorure de polyvinyle (PVC), which offers excellent plasticity, transparence, and barrier properties at a low cost. Some drugs with high barrier requirements (such as tablets that are susceptible to moisture absorption and oxidation) may use polyvinylidene chloride (PVDC)-coated PVC, or polyamide (Pennsylvanie, nylon), polypropylène (PP), etc., to enhance moisture and oxygen resistance.
– Lid (a thin sheet sealed to the blister shell)
Purpose: Seals the blister, preventing drug leakage and external factors from entering.
Common Materials:
Feuille d'aluminium: The most common, offering excellent barrier properties (humidité, oxygène, and light shielding) and easy tearing. The surface is usually coated with an adhesive to ensure adhesion to the blister shell.
Composite films: Such as aluminum-plastic composite films (feuille d'aluminium + film plastique) are suitable for applications requiring flexibility.
avantages:
– Provides excellent protection, effectively shielding tablets from external environmental influences such as moisture and light.
– Ensures the sealing and integrity of the tablet packaging, preventing contamination or deterioration.
– Makes it easy for patients to view and remove tablets. The transparent blister allows them to visually identify the appearance and quantity of the tablets.
– Blister packaging often has anti-counterfeiting features, effectively preventing drug substitution or counterfeiting.
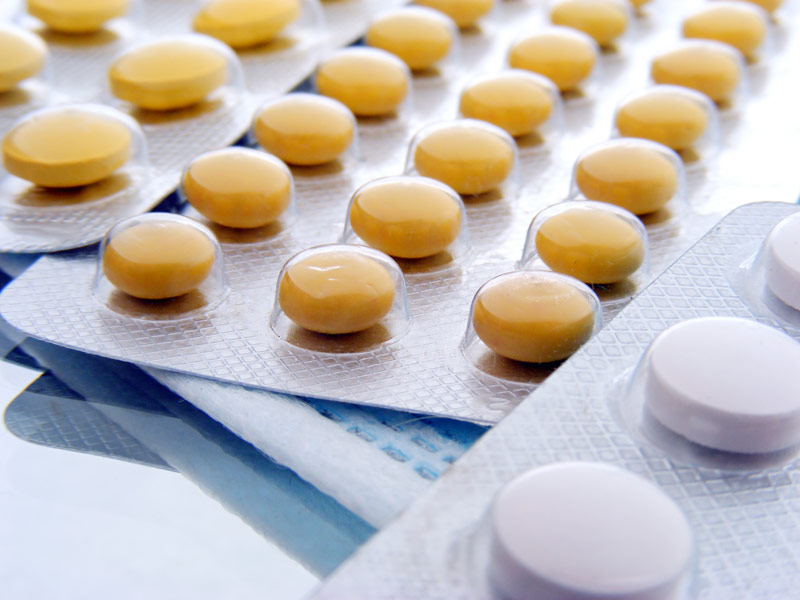
Strip packaging, also known as stick packaging, features a structure in which tablets are sandwiched between two layers of packaging material. Heat-sealing the edges creates a continuous, independent unit (each unit contains one or more tablets). The overall unit is in the form of a strip or tape and can be cut into individual strips as needed.
Structural Components and Materials:
Packaging Material (Two-Layer Composite Film)
The core of the strip packaging is two layers of heat-sealable film. The tablets are wrapped in the middle. Heat sealing tightly bonds the two layers around the tablets, forming a sealed unit. Common Material Combinations:
– Feuille d'aluminium + Feuille d'aluminium: This two-layer aluminum foil composite offers strong barrier properties (résiste à l'humidité, l'oxygène à l'épreuve, and light-blocking) and sealing properties. It’s suitable for tablets requiring high stability, but it’s also more expensive and stiff, requiring a firm tear to open.
– Feuille d'aluminium + Plastic Film (par exemple., PE, PP): The aluminum foil provides barrier properties, while the plastic film provides heat-sealing properties and a certain degree of flexibility. This combination offers balanced overall performance at a moderate cost, making it a more commonly used combination.
– Plastic Film + Plastic Film: Par exemple, a composite of PET and PE offers weaker barrier properties than the aluminum foil combination. It’s suitable for tablets with high stability, lower cost, and offers greater flexibility and ease of opening.
Heat-Sealed Edge: The heat-sealed portion of the two materials around the tablet creates a sealed boundary, preventing air and moisture from entering while separating each tablet unit for independent storage.
avantages:
– Strip packaging can increase pharmaceutical packaging speed and efficiency, making it suitable for large-scale tablet packaging.
– It can reduce pharmaceutical packaging labor costs, améliorer l'efficacité de la production, and lower packaging costs. – Strip packaging can be customized in various sizes and shapes, suitable for tablet packaging of varying sizes.
– It facilitates packaging and sealing operations, reducing human error and damage during the packaging process.
3. Emballage blister en aluminium et plastique
Similar to blister packaging, it emphasizes the tightness of the aluminum-plastic composite. It is commonly used for tablets requiring higher barrier properties (such as sustained-release and controlled-release tablets).
Structure and Materials:
Blister Shell: Typically made of PVC / PVDC composite film or PP/PA composite film to enhance barrier properties.
Lid Material: Thick aluminum foil (thicker than standard blister lids) enhances sealing and puncture resistance, making it suitable for medications requiring higher protection.
4. Pouch Packaging
Less commonly used for tablets (mostly granules and powders), but may be used for small-dose tablets (tel que 1-2 tablets of emergency medicine). The structure is a composite pouch. Materials:
Common composite film materials include aluminum-plastic composite film (feuille d'aluminium + PE/PP) and aluminized film (such as aluminized PET + PE). These films offer certain barrier and sealing properties, are lightweight, and are easy to carry.
En général, blister packaging is suitable for pharmaceutical packaging that requires enhanced protection and display, while strip packaging is suitable for high-volume production, improving production efficiency and reducing costs. Le choix de emballage de comprimés pharmaceutiques structure and material is primarily based on a combination of factors, including tablet stability requirements (par exemple., humidité, lumière, and oxidation sensitivity), shelf life, usage scenarios (par exemple., portability), et le coût. The core objective is to ensure consistent quality throughout the product’s shelf life.